Holliston, MA – November 12, 2019 – Biostage, Inc. (OTCQB: BSTG), a bioengineering company developing next-generation esophageal implants, today announced its financial results for the three and nine months ended September 30, 2019.
During the third quarter, Biostage continued its focus to complete the submission of its first Investigational New Drug (IND) application.
On October 29, 2019, Biostage submitted its IND application to the U.S. Food and Drug Administration (FDA) for the Company’s lead product candidate, the Cellspan™ Esophageal Implant (CEI).
Biostage CEO James McGorry remarked, “This milestone marks the start of Biostage’s transition to a clinical stage company and, subject to FDA approval, will allow Biostage to begin clinical trials of our Cellspan Esophageal Implant. Implantation of this bioengineered tissue during surgical resection of a short segment of the esophagus addresses important and unmet public health needs, those of adults as well as children. This initial clinical trial will enroll adults and will enable a subsequent regulatory filing for a pediatric-focused clinical trial. Biostage’s technology has the potential to enable patients to avoid multiple complex surgeries, while also improving the patient’s quality of life compared to the current standard of care.”
Mr. McGorry continued, “The CEI is innovative in its composition and utility, while also providing surgeons with a new option that holds the promise of reducing morbidity both short and near-term.”
Third Quarter Operating Highlights
During the third quarter of 2019, Biostage continued to advanced its operating programs aimed at bringing its potentially life-changing Cellspan technology to underserved patient populations. Biostage also received approximately $1.2 million from the exercise of warrants during the quarter, extending the Company’s finances and demonstrating investor confidence in the Company’s technology.
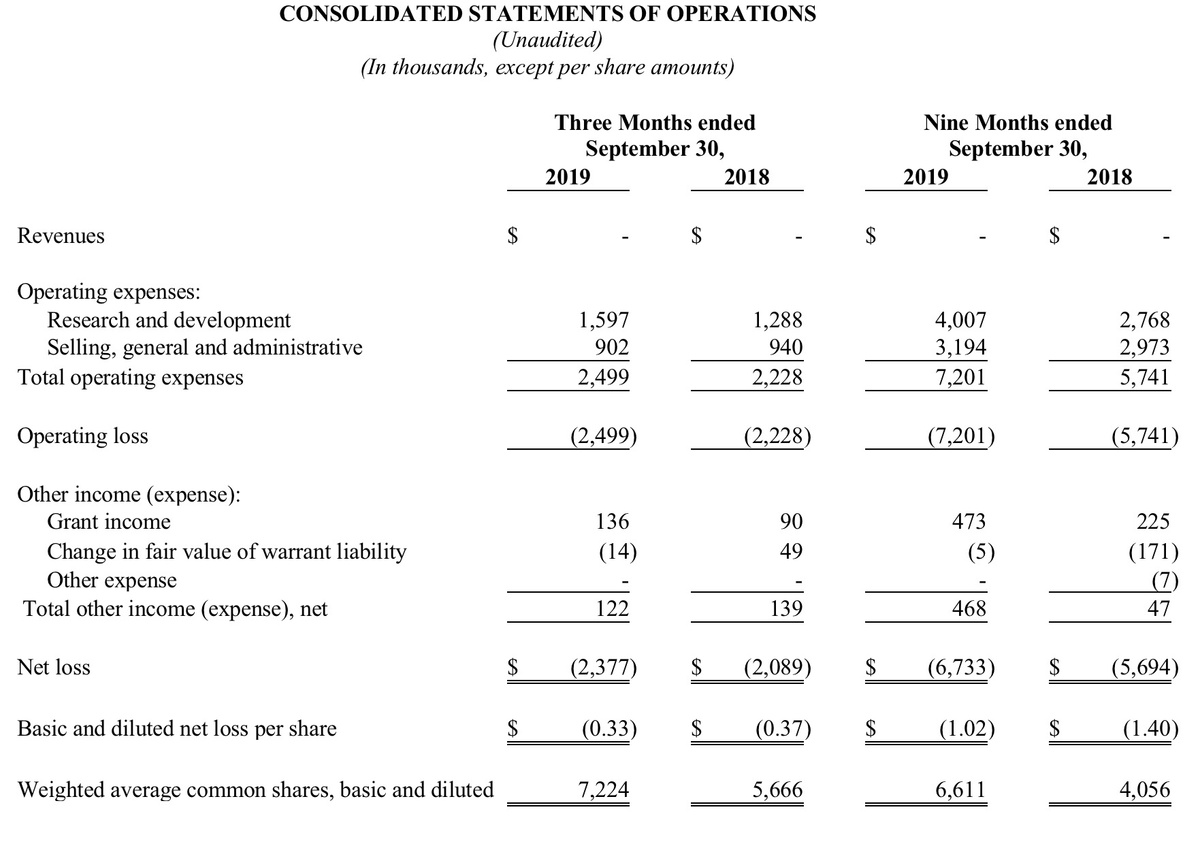
Summary of Financial Results
For the three months ended September 30, 2019, the Company reported a net loss of $2.4 million, or a net loss per share of $0.33, compared to a net loss of $2.1 million, or a net loss per share of $0.37 for the three months ended September 30, 2018. The $0.3 million year-over-year increase in net loss was due primarily to a $0.3 million increase in research and development costs. General and administrative expenses and other income (expense) for the current quarter were virtually unchanged compared to the same period last year. In addition, the Company recognized grant income for qualified expenditures from the SBIR grant of $136,000 for the current three-month period compared to $90,000 for the similar three-month period in 2018.
For the nine months ended September 30, 2019, the Company reported a net loss of $6.7 million, or a net loss per share of $1.02, compared to a net loss of $5.7 million, or a net loss per share of $1.40 for the nine months ended September 30, 2018. The $1.0 million year-over-year increase in net loss was due primarily to a $1.2 million increase in research and development costs and a $0.2 million increase in general and administrative expenses, offset in part by a $0.2 million net decrease in expense from change in the fair value of warrants. In addition, the Company recognized grant income for qualified expenditures from the SBIR grant of $473,000 for the current nine-month period compared to $225,000 for the similar nine-month period in 2018.
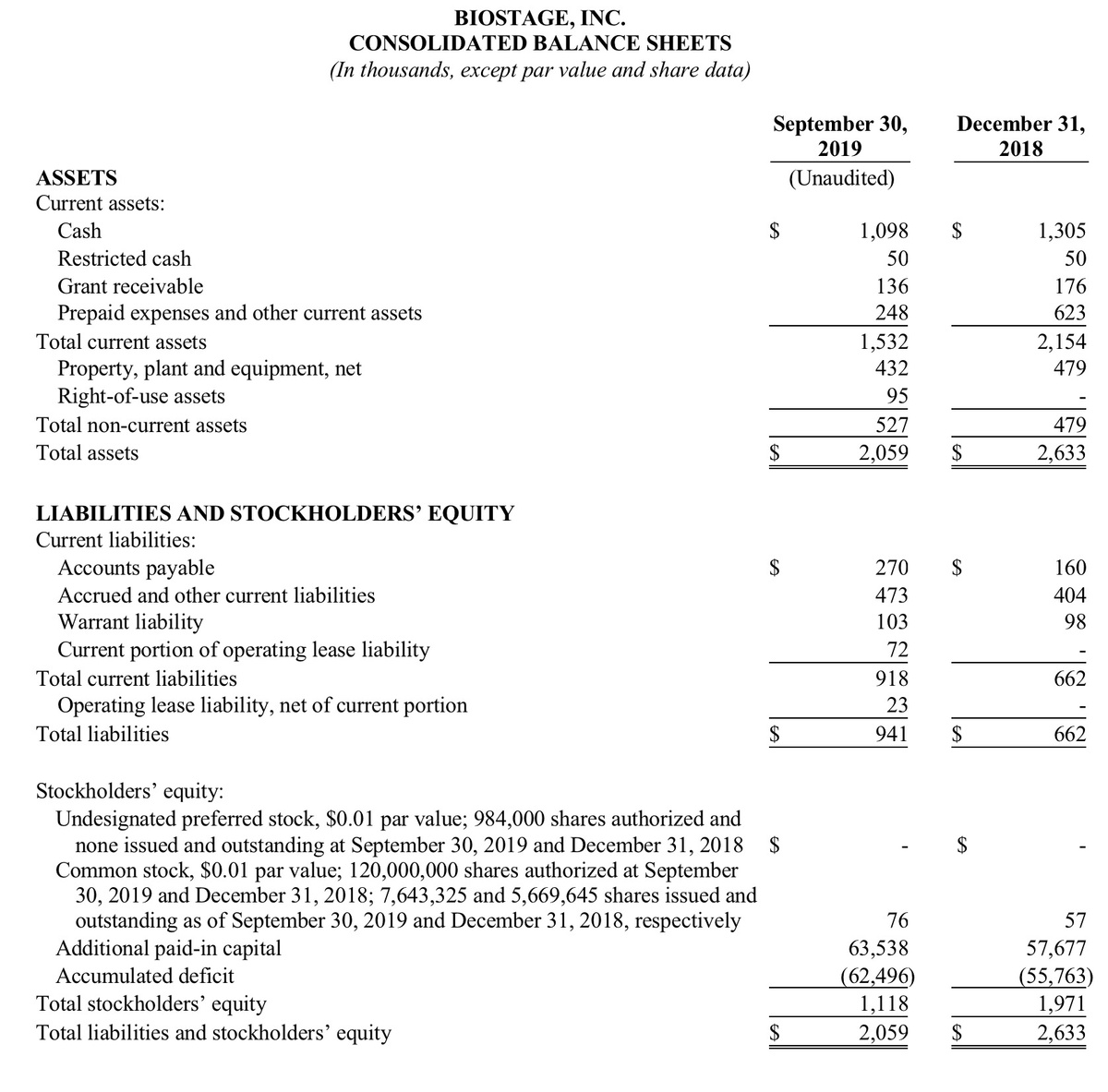
Balance Sheet and Cash
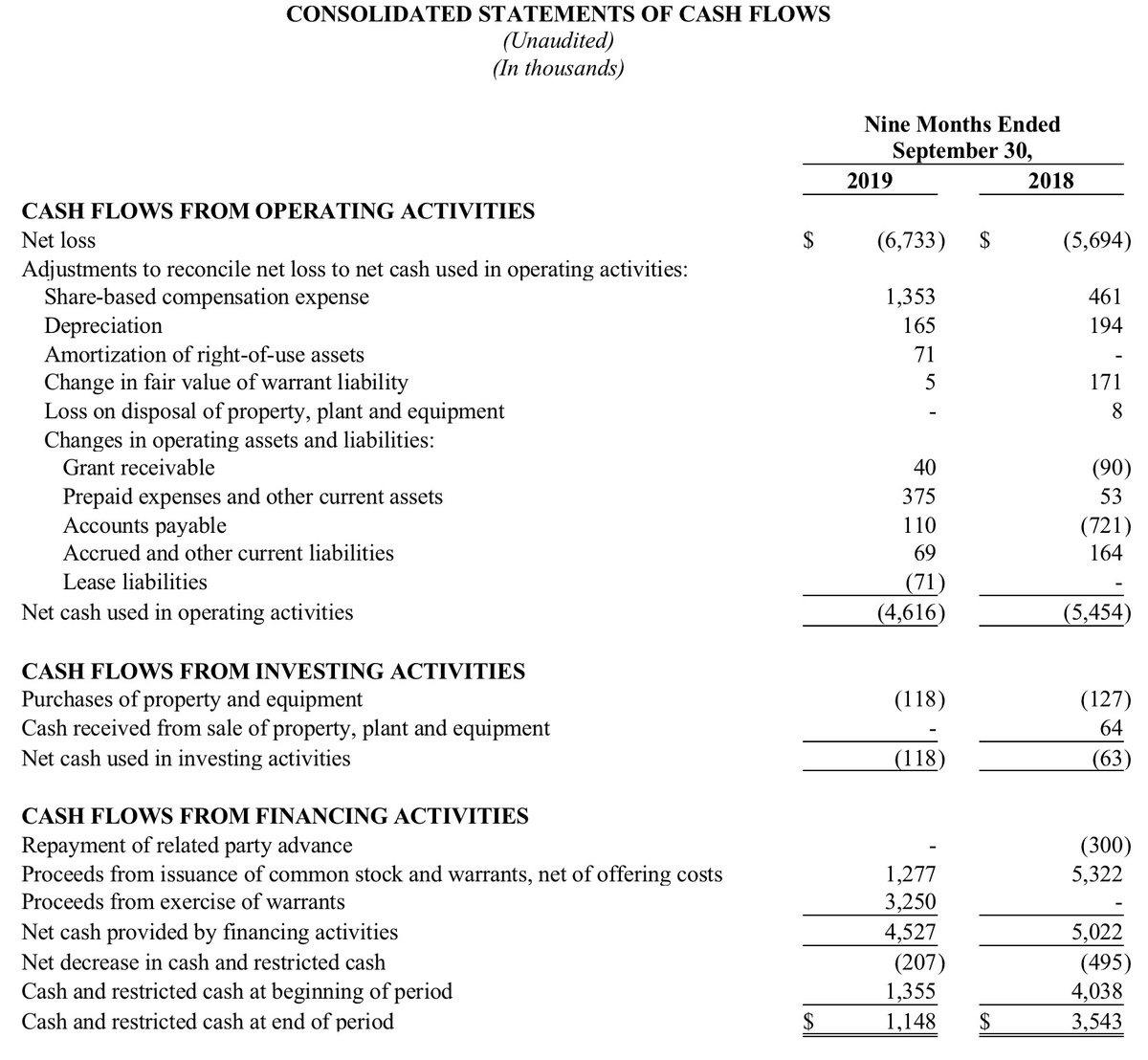
At September 30, 2019, the Company had cash on-hand of $1.1 million and no debt. The Company used net cash in operations of $4.6 million during the nine months ended September 30, 2019.
During the nine months ended September 30, 2019, the Company received $4.5 million from financing activities, including approximately $1.3 million from the issuance of 345,174 shares of common stock to investors in private placement transactions, and $3.25 million from the issuance of 1,625,000 shares of its common stock to a group of investors in connection with the exercise of a portion of the warrants that were issued on December 27, 2017.
Subsequent to the end of the quarter, the Company issued an additional 75,000 shares of common stock to Connecticut Children’s Medical Center (Connecticut Children’s) in exchange for the exercise of warrants, which were previously issued to Connecticut Children’s on January 3, 2018. Such warrants were exercised for aggregate cash proceeds of $150,000.
Call-in Information
Biostage will be hosting a conference call and webcast today at 9:00 am ET to review its operational progress and financial report. On that call, management may respond to questions from the audience and provide information on any of a number of topics related to the business.
To participate in the call, please dial (877) 407-8293 (domestic) or (201) 689-8349 (international). The live audio webcast will be accessible on the Events page of the Investors section on the Company's website at www.biostage.com, and will be archived for 60 days. An audio webcast will be available for one week following the call and can be accessed during that period by dialing (877) 660-6853 (domestic) or (201) 612-7415 (international) with Conference ID #:13696581.
About Biostage, Inc.
Biostage is a bioengineering company that is developing next-generation esophageal implants. The Company’s Cellspan technology combines a proprietary, biocompatible scaffold with a patient’s own cells to create an esophageal implant that could potentially be used to treat pediatric esophageal atresia and other conditions that affect the esophagus. The Company’s esophageal implant leverages the body’s inherent capacity to heal itself as it is a “living tube” that facilitates regeneration of esophageal tissue and triggers a positive host response resulting in a tissue-engineered neo-conduit that restores continuity of the esophagus. These implants have the potential to dramatically improve the quality of life for children and adults. At Biostage, we believe the future of medicine has been inside us all along.
For more information, please visit www.biostage.com and connect with the Company on Twitter and LinkedIn.
Forward-Looking Statements
Some of the statements in this press release are "forward-looking" and are made pursuant to the safe harbor provision of the Private Securities Litigation Reform Act of 1995. These "forward-looking" statements in this press release include, but are not limited to, statements relating to our financing activities; development expectations and regulatory approval of any of the Company’s products, including those utilizing its Cellspan and Cellframe™ technology, by the U.S. Food and Drug Administration, the European Medicines Agency or otherwise, which expectations or approvals may not be achieved or obtained on a timely basis or at all; or success with respect to any collaborations, clinical trials and other development and commercialization efforts of the Company’s products, including those utilizing its Cellspan and Cellframe technology, which such success may not be achieved or obtained on a timely basis or at all. These statements involve risks and uncertainties that may cause results to differ materially from the statements set forth in this press release, including, among other things, the Company’s inability to obtain needed funds in the immediate future; the Company’s ability to obtain and maintain regulatory approval for its products; plus other factors described under the heading "Item 1A. Risk Factors" in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2018 or described in the Company’s other public filings. The Company’s results may also be affected by factors of which the Company is not currently aware. The forward-looking statements in this press release speak only as of the date of this press release. The Company expressly disclaims any obligation or undertaking to release publicly any updates or revisions to such statements to reflect any change in its expectations with regard thereto or any changes in the events, conditions or circumstances on which any such statement is based.